What is it?
The GenoImmu™ COVID-19 Antigen Detection Kit employs immuno-lateral chromatography technology for the qualitative detection of antigens.
The colloidal gold particles labelled with the anti-SARS-CoV-2 antibody 1 are fixed on the conjugation pad. The anti-SARS-CoV-2 antibody 2 is bound on the “T” test line of nitrocellulose membrane. The goat anti-mouse IgG is bound on the “C” control line of nitrocellulose membrane.
When the concentration of SARS-CoV-2 in the specimen is higher than the minimum detection limit, which can conjugate with the anti-SARS-CoV-2 antibody 1 labelled with colloidal gold particles to form a complex. This complex migrates on the membrane via capillary action until the test line, where it will be captured by the anti-SARS-CoV-2 antibody 2 bound on the test line, forming “Au-anti-SARS-CoV-2 antibody 1-(SARS-CoV-2) -anti-SARS-CoV-2 antibody 2 complex. These complexes are deposited to display colour as the determination of antigen positive, the rest of anti-SARS-CoV-2 antibody 1 labelled with colloidal gold particles conjugate with the goat anti-mouse IgG and deposit to display colour as the determination of quality of the “C” control line.
When the concentration of SARS-CoV-2 in the specimen is lower than the minimum detection limit or no SARS-CoV-2, the complexes only deposit and display colour in the “C” control line.
Who is it for?
The product is for in vitro diagnostics use only. The test should be used for the detection of SARS-CoV-2 antigen in oropharyngeal swab, nasopharyngeal swab and anterior nasal swab specimens only.
It is for laboratory personnel who have received professional guidance or training and have professional knowledge of in vitro diagnosis, also for relevant personnel who have received infection control or nursing training.
The test results of the test kits are for clinicians' reference only, and should not be used as the only basis for clinical diagnosis. The clinical management of patients should be comprehensively considered in combination with their symptoms / signs, medical history, other laboratory tests and treatment responses, etc.
Why is it different?
The test strip is compatible with multiple sample types, including oropharyngeal swab, nasopharyngeal swab and anterior nasal swab specimen in vitro. It has ≥ 96% sensitivity and ≥ 99% specificity in all compatible sample types.
Advantages
Fast – Result in 15 – 20 minutes
Easy – User-friendly with a simple visualization result
Accurate – Sensitivity ≥ 96%, Specificity ≥ 99%
Cost effective – Competitively priced
Complete – Able to detect SARS-CoV-2 variants such as Delta and Omicron
Need to know
This is a consumable assay kit, validated, supplied and supported by DiagCor.
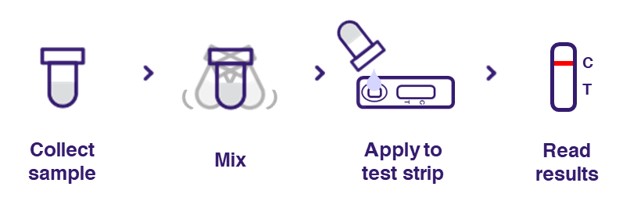
- Workflow
This rapid workflow is for the GenoImmuTM COVID-19 Antigen Detection Kit. Please refer to the product IFU for the detailed test procedure, as the exact sample collection and handling procedure depends on the sample type.