
Please check your inbox.

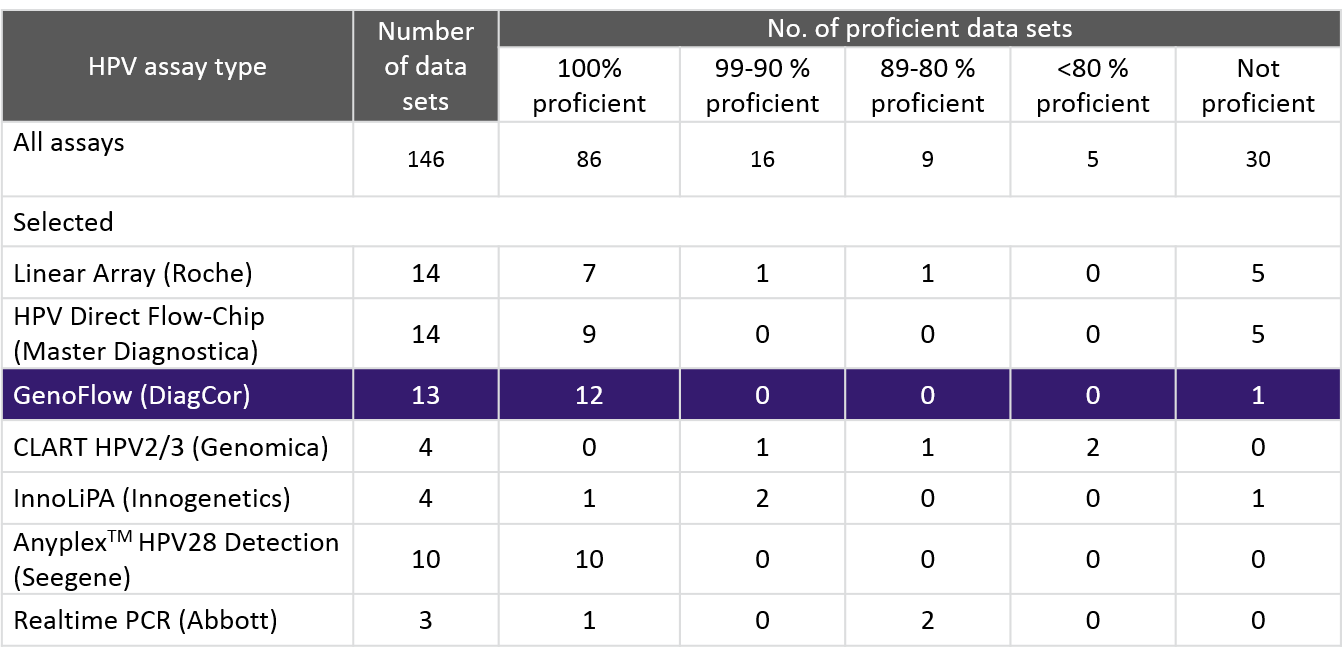
In recent study of 2014 WHO HPV LabNet proficiency, DiagCor scored 12 labs with 100% proficiency.
The WHO HPV LabNet has developed this international proficiency panel for HPV DNA detection and typing, and organized annual proficiency studies since 2007. The 2008 WHO HPV LabNet Proficiency Study for Evaluating HPV DNA Typing Methods was open for global participation for all interested laboratories to evaluate different HPV tests in different laboratories.
DiagCor’s GenoFlow HPV array test detects 33 HPV subtypes (Including 17 high-risk subtypes and 16 low-risk subtypes). The kit will also identify the presence of HPV molecules other kits might miss adopting Universal Probe. In 2010, 2011 and 2013 WHO HPV LabNet studies, DiagCor also obtained 100% proficiency.
Reference:
Eklund C et al. The 2010 Global Proficiency Study of Human Papillomavirus Genotyping in Vaccinology. J Clin Microbiol (2012) 50(7): 2289-98
About DiagCor
Headquartered in Hong Kong with ISO 13485 accredited Quality Management System, DiagCor has been dedicated solely to molecular diagnostics (MDx) since 2006. The global leader in flow-through hybridization technology, the company offers a range of products, solutions and services related to RNA and DNA analysis. As well as providing MDx laboratory services to Asia Pacific practitioners, DiagCor develops and manufactures MDx products and solutions, and offers MDx medical translation services and MDx consulting.
Press Contact
DiagCor Life Science